Show your work for the calculation of empirical formula here Excessive physical activity, lactic acid molecular mass 90.08 g per mole, forms in muscle tissues and is responsible for muscle soreness. Elemental analysis shows that this compound has 40.0% carbon 6.71% hydrogen and 53.3% oxygen. Determine the empirical formula of lactic acid.. 6) A hydrocarbon (a compound that contains only hydrogen and carbon) is found to be 74.5 % carbon by mass. a. Find the empirical formula for the compound. b. If the molar mass of the compound is 16.05 g/mol, find its molecular formula. 7) It was found that a compound contained 68.1% carbon, 13.7% hydrogen, and 18.2% oxygen by mass.

Chemistry Empirical And Molecular Formula Worksheet Answers

1 Empirical and Molecular Formula 1 Empirical and Molecular Formula Worksheet SHOW WORK ON

12 Best Images of Empirical Formula Worksheet With Answers Molecular and Empirical Formula

Determining Empirical Formulas Worksheet Printable Word Searches

Free Empirical/molecular Formula Practice Worksheets

Empirical and Molecular Formula Notes Chemistry Classes / Ronald Reagan S.H.S.

Solved Empirical and Molecular Formula Worksheet SHOW WORK
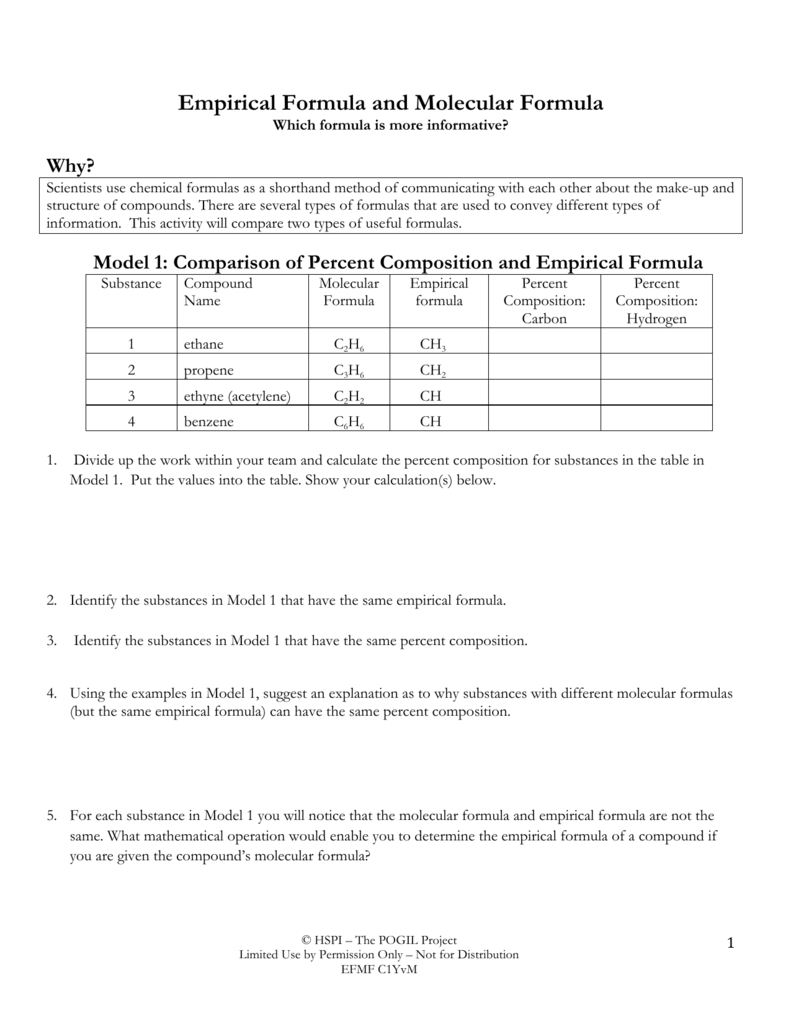
Empirical Formula and Molecular Formula

Empirical Formula Molecular Formula Worksheet Worksheets For Kindergarten

Calculating Empirical Formula Worksheet CALCULATOR GHW

6 Problems Worksheet Empirical and Molecular Formulas

Empirical/Molecular Formulas
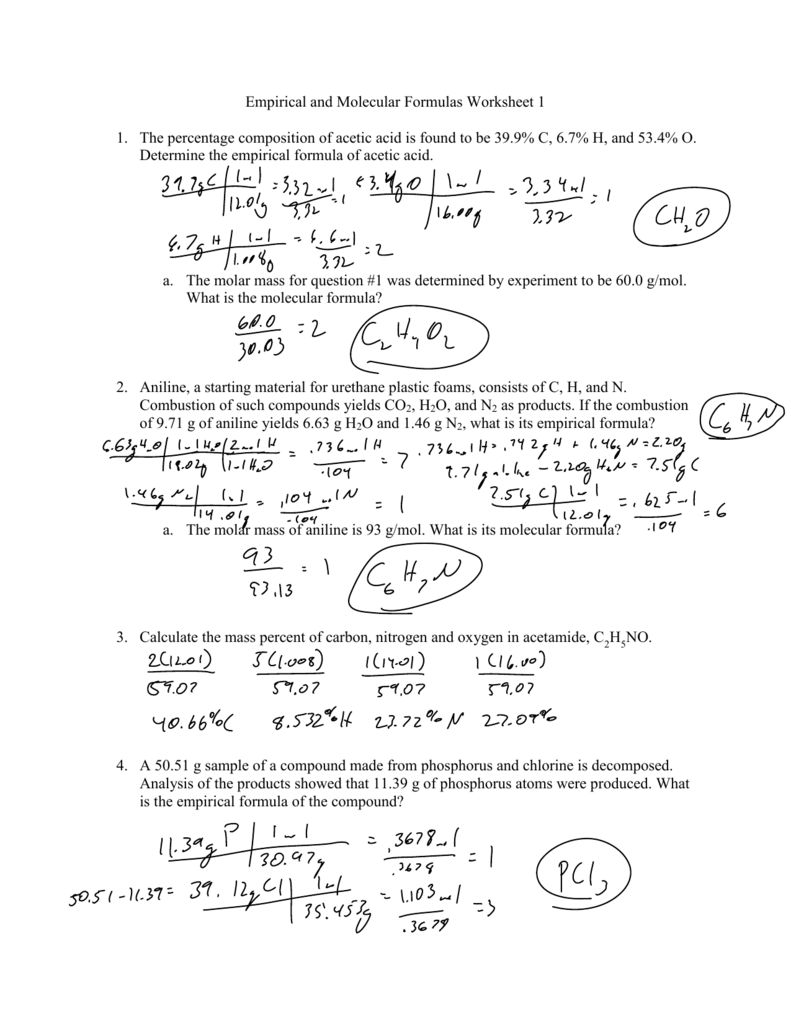
Empirical and Molecular Formulas Worksheet 1 1. The percentage

Empirical Formula Worksheet Free Worksheets Samples
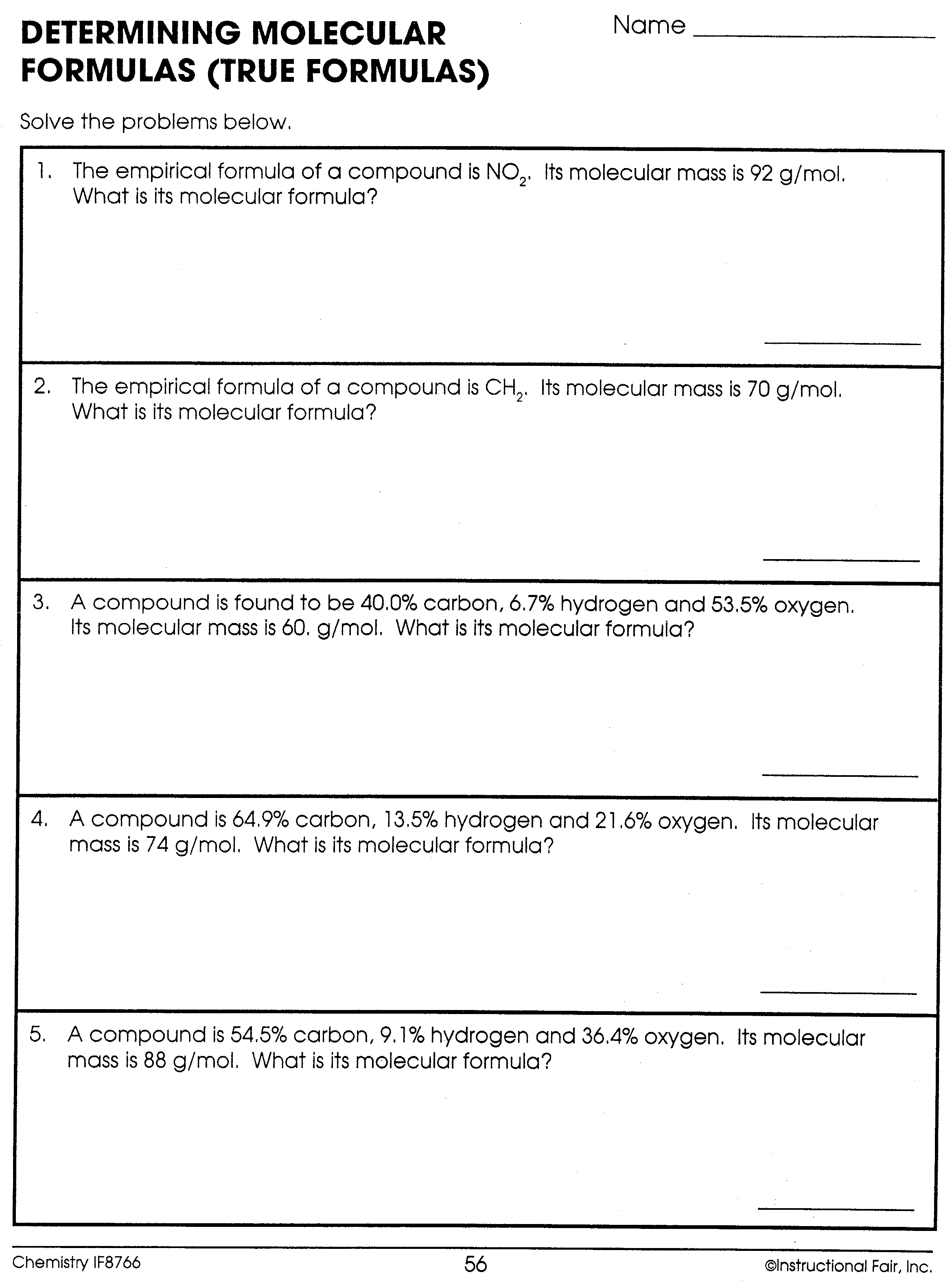
Empirical And Molecular Formula Problems Pdf UPDATED

Free Empirical/molecular Formula Practice Worksheets
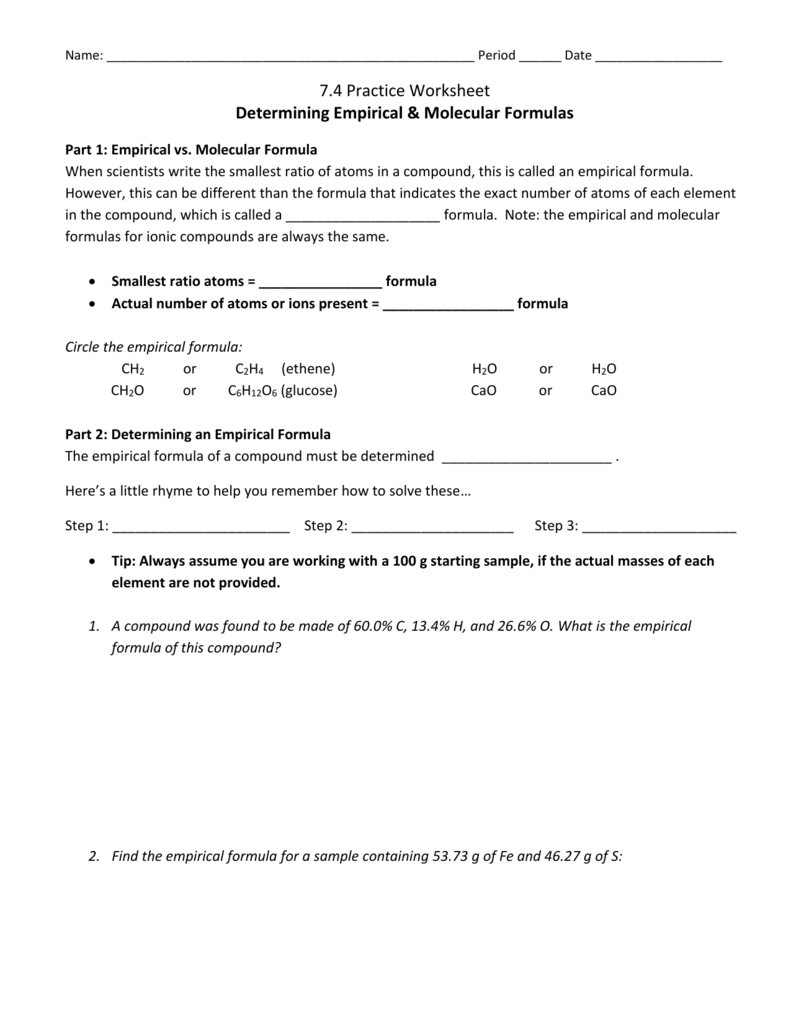
Determining Empirical & Molecular Formulas

Free Empirical/molecular Formula Practice Worksheets

13 Best Images of Covalent Formulas Worksheet Writing Ionic Compound Formula Worksheet Answers

Empirical And Molecular Formula Worksheet Answer Key —
The empirical formula for this compound is thus CH 2. This may or may not be the compound's molecular formula as well; however, additional information is needed to make that determination (as discussed later in this section). Consider as another example a sample of compound determined to contain 5.31 g Cl and 8.40 g O.. A 100.0g piece was analyzed and found to have 66.5g Cu combined with 33.5g S. To find the empirical formula: 1. Convert from mass to moles using the MM of the element. 2. Repeat for all elements in the compound. 66.5 g Cu x 1 mol Cu = 1.05 mol Cu 33.5 g S x 1 mol S = 1.05 mol S. 63.55 g Cu.